ProKidney Drives a Truly Advanced Cell Therapy for Diabetic CKD
ProKidney Drives a Truly Advanced Cell Therapy for Diabetic CKD
🔹 Executive Company Summary – ProKidney Corp.
Ticker: NASDAQ: PROK | Sector: Cell Therapy / Regenerative Medicine
Headquarters: Winston-Salem, North Carolina, USA
CEO: Tim Bertram, PhD
Employees: ~100 (estimated)
Website: www.prokidney.com
Regulatory Designations: RMAT (FDA) | Orphan Drug (EU, prior)
Product Model: Autologous cell therapy administered directly into the kidney
Pipeline:
REACT (Rilparencel) – autologous cell therapy currently in Phase 3 for diabetic CKD
Proprietary platform for expansion of adult kidney-derived cells
Ongoing regulatory alignment in the U.S., with potential expansion into the EU
Summary:
ProKidney Corp. is a biopharmaceutical company focused exclusively on autologous cell therapies for chronic kidney disease (CKD). Its lead candidate, REACT, utilizes kidney-derived cells collected from the patient, expanded ex vivo, and reinjected directly into the renal cortex. The therapy is designed to regenerate nephron structure and function, targeting structural preservation and delay of end-stage renal disease. With RMAT designation from the FDA and a pivotal Phase 3 trial underway, ProKidney’s model proposes a regenerative and durable approach in contrast to conventional systemic therapies.
🔬 How REACT Works
A kidney biopsy is performed to collect cortex tissue.
Key progenitor cells (podocytes, mesenchymal, ureteral) are expanded ex vivo.
The cells are cryopreserved and later reinjected into the renal cortex under imaging guidance.
These cells integrate and release regenerative signals (anti-fibrotic, anti-inflammatory, erythropoietin, active vitamin D3), promoting glomerular and tubular recovery.
📊 Key Results: Phase 2 – REGEN‑007
Arm 1 (n=24): 78% improvement in annual eGFR decline – statistically and clinically significant
Arm 2 (n=25): 50% improvement trend, but not statistically significant
Safety: No serious adverse events related to REACT; risk profile comparable to renal biopsy
⚖️ Regulatory Positioning
RMAT designation granted by FDA
Phase 3 Trial (PROACT 1): Sham-controlled, ~685 patients, composite endpoint: ≥40% eGFR decline, dialysis initiation, transplant, or renal/CV death
Type B Meeting with FDA (Summer 2025): Will determine whether eGFR slope qualifies as a surrogate endpoint for accelerated approval
Full Phase 2 data to be presented at ASN Kidney Week 2025
Note: The regulatory acceptability of eGFR slope as a standalone surrogate endpoint remains conditional. The FDA has previously signaled openness through RMAT discussions, but no formal validation has yet been granted.
eGFR slope has been recognized as a "reasonably likely" surrogate endpoint in CKD by regulatory science working groups such as NKF–FDA–KDIGO (Inker et al., CJASN 2021).
🧪 Trial Phase Context & Methodological Caveats
Phase 2 clinical trials are designed to assess biological activity, dose optimization, and safety in a limited patient population. They are not definitive studies.
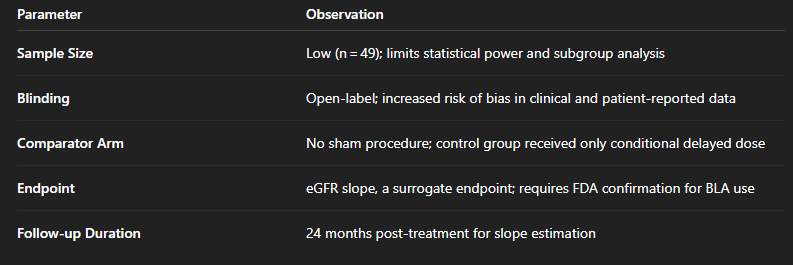
Key limitations of REGEN-007 include:
While the observed +4.6 mL/min/year improvement is biologically striking, inter-patient variability in late-stage CKD and the lack of blinding warrant caution. These results should be considered hypothesis-generating and not yet generalizable to the broader diabetic CKD population.
📈 Market Response – NASDAQ: PROK
+500% price surge after Phase 2 announcement: US$0.64 → US$3.73 intraday
Over 343 million shares traded in a single session
Analyst sentiment:
Citi: Buy, target US$9
Bank of America: Underperform, citing lack of sham and endpoint uncertainty
Evercore ISI: In-Line, recognizes signal but notes regulatory and timeline risk
🔚 BBIU Conclusion
ProKidney advances with a rigorously structured regulatory plan and early-phase data that suggest real nephron-preserving potential in diabetic CKD. However, pivotal results from Phase 3 and definitive FDA positioning on eGFR slope will determine the true commercial trajectory. As it stands, REACT is among the few regenerative therapies targeting chronic diseases with durable outcomes — not just biochemical modulation.