Biopharma – Medicine
Where Science Becomes Narrative—and Narrative Becomes Policy
This section dissects the bio-medical system not as a scientific endeavor, but as a symbolic engine where regulation, language, and power converge.
We trace how clinical data becomes currency, how truth fragments through institutional layers, and how health is reframed as alignment to protocol.
In medicine today, what is authorized is not always what is true.
![🟡 [FDA Debate Over Fluoride Supplements for Children]](https://images.squarespace-cdn.com/content/v1/685a879d969073618e9775db/1753410105620-7LZN7YI3NNXHGHP6XTW1/istockphoto-1160757152-612x612.jpg)
🟡 [FDA Debate Over Fluoride Supplements for Children]
While fluoride’s protective role in dental health is well-established, particularly through topical applications, the systemic ingestion model—especially in children—raises unresolved questions. The current FDA debate exposes a deeper structural tension: a product exempt from modern approval standards seeks renewed legitimacy under the banner of equity, despite low systemic efficacy and unclear long-term safety. The promotion of fluoride tablets reflects not only gaps in scientific consensus but also the persistence of symbolic public health interventions that may serve industrial supply chains more than patient needs. When effective topical alternatives exist, the systemic route becomes not a medical necessity, but a strategic and symbolic decision.
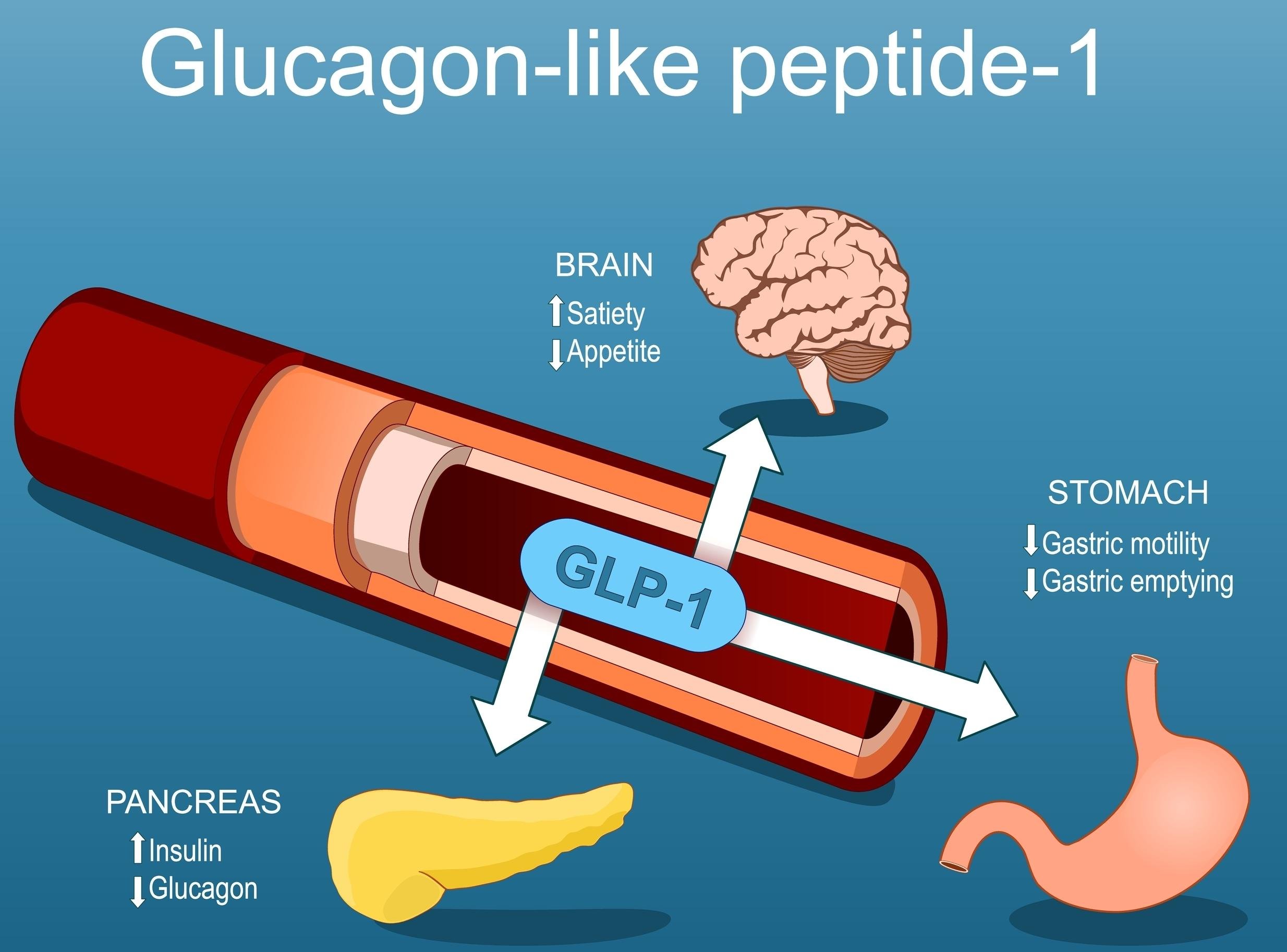
Evaluating the Risk of Lean Mass Reduction During GLP-1 Receptor Agonist Therapy
“While GLP-1 receptor agonists have revolutionized the treatment of metabolic disease, the emerging pattern of lean mass loss—accounting for up to 40% of total weight reduction in some trials—raises a clinically relevant paradox. Weight is lost, but so is structure. Without proactive intervention, this silent catabolism may compromise physical function, metabolic resilience, and quality of life. The therapeutic conversation must now evolve: from weight loss alone to functional preservation.”

🔎 FDA Rejects Brexpiprazole–Sertraline Combo for PTSD: Signal or Systemic Failure?
“Despite a mechanistically compelling rationale and one statistically positive Phase 3 trial, the FDA decisively rejected the brexpiprazole–sertraline combination for PTSD due to inconsistent efficacy and regulatory standards requiring replication. This case exposes the structural fragility of psychiatric drug development, where even a ~5-point improvement on CAPS-5 is insufficient without reproducibility. It also underscores a broader tension: between the urgent need for new PTSD treatments and the rigid evidentiary bar that neuropsychiatric indications must clear.”

Symbolic Biomarkers of Cognitive Decline: TEI and EV as Non-Invasive Diagnostic Tools in Medicine
Traditional diagnostic tools often fail to detect early structural changes in cognition. This paper introduces TEI (Token Efficiency Index) and EV (Epistemic Value)—originally designed to evaluate symbolic integrity in human–AI interactions—as non-invasive, language-agnostic biomarkers for assessing cognitive decline and psychiatric disorganization. By quantifying coherence, domain activation, and logical verifiability in natural language, these symbolic metrics provide real-time insight into neurocognitive integrity. Potential applications span early-stage dementia, factitious disorders, schizophrenia, and other conditions where cognitive architecture degrades before memory loss becomes measurable. TEI and EV offer a scalable, objective layer to modern neuropsychiatric evaluation.

From Petrochemicals to Paracetamol: Korea’s Strategic Leap Toward Circular Bio-Industry
South Korea’s petrochemical industry stands at a critical crossroads. Global oversupply, ESG pressure, and waning competitiveness are forcing a reckoning. But within this crisis lies an untapped industrial opportunity: transforming PET plastic waste into pharmaceutical-grade paracetamol, clean energy, and infrastructure-grade materials.
This article outlines a techno-biological roadmap to reposition Korea’s chemical sector as a global circularity leader—merging green chemistry, synthetic biology, and industrial-scale waste valorization. If executed, it could not only yield financial returns within five years but redefine Korea’s industrial identity for the post-fossil era.
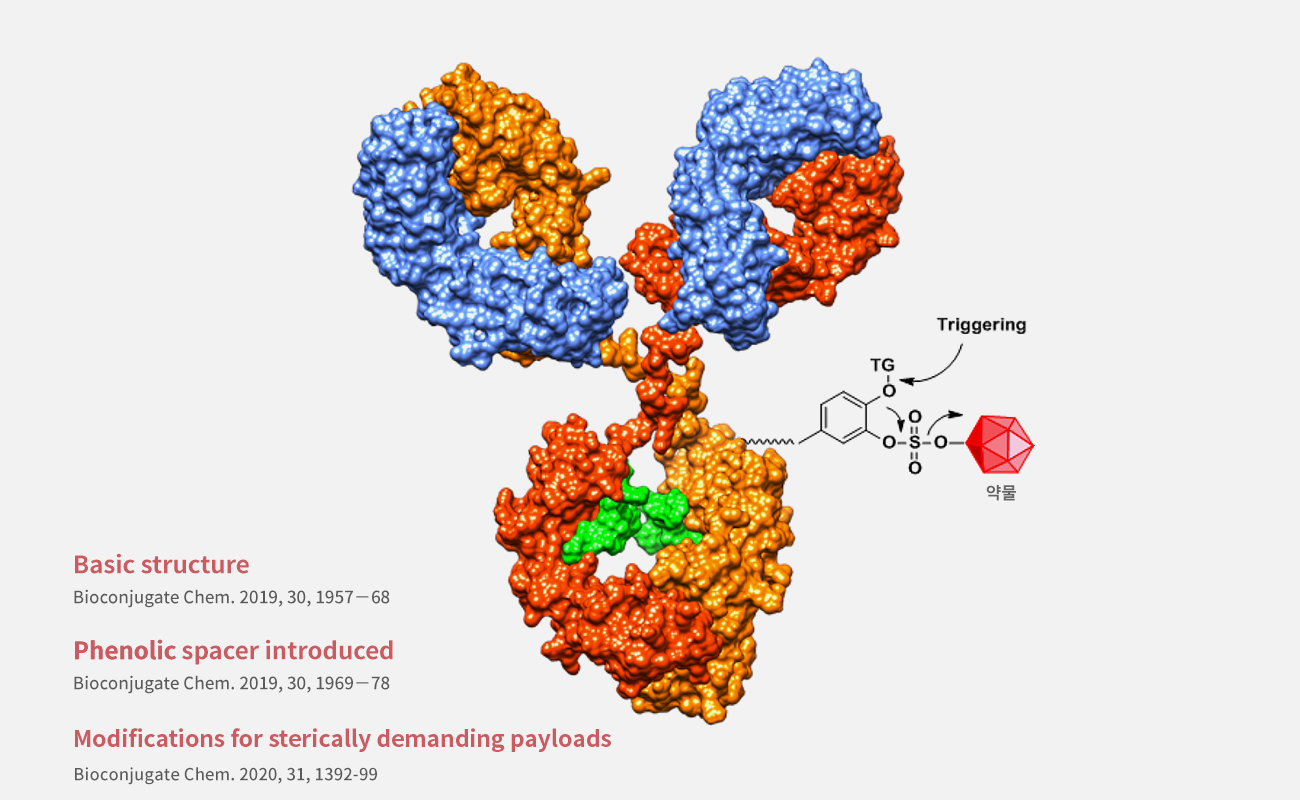
The Hidden Patent Threat Undermining IntoCell’s Nexatecan PlatformA forensic patent analysis following ABL Bio’s sudden technology withdrawal
When ABL Bio abruptly returned the Nexatecan platform to IntoCell, few expected the root cause to lie beneath international waters — a Chinese submarine patent with striking structural similarities. This forensic comparison exposes the overlap between US20220047717A1 and WO2023088235A1, raising critical questions about freedom to operate, IP governance, and the hidden risks lurking in ADC development pipelines.

ProKidney Drives a Truly Advanced Cell Therapy for Diabetic CKD
Can an autologous cell therapy shift the course of diabetic kidney disease?
ProKidney’s REACT (Rilparencel) delivers a striking signal: a +4.6 mL/min/year improvement in eGFR slope in a Phase 2 study — one of the strongest functional preservation outcomes ever recorded in late-stage CKD.
But beneath the excitement lies a complex regulatory and methodological landscape. With no sham control, small sample size (n=49), and reliance on a surrogate endpoint, the data must be interpreted with precision.
This BBIU report dissects the trial design, FDA alignment, market reaction (NASDAQ: PROK +500%), and strategic outlook. It also frames the risks: regulatory uncertainty, lack of long-term durability data, and the challenge of scaling autologous manufacturing.
REACT is not a promise — it's a possibility. One that, if validated in Phase 3, could redefine what is therapeutically achievable in diabetic CKD.

Adipose-Derived Stem Cell Bioink for Facial Rejuvenation: A Regenerative Strategy Post-CO₂ Laser Resurfacing
“What if we could turn the downtime of CO₂ laser into a window for biologically driven regeneration? By applying an autologous, stem-cell enriched bioink through a dermal patch immediately post-resurfacing, we propose a method to reduce recovery time by up to 60% while enhancing collagen remodeling and patient satisfaction. This is not just skin care — it’s strategic immune-guided healing.”

Ocular Bioink as an Immunoregenerative Interface: An Open Proposal from the Clinical Frontier
We propose a modular therapeutic platform combining autologous immune-derived and stem cell–based bioinks, printed onto scleral lenses, to simultaneously control viral/inflammatory activity and promote corneal healing. This immunoregenerative interface could prevent permanent visual impairment from post-infectious scarring and reduce the economic burden of corneal transplantation. It is a call to co-develop translational solutions that reshape how ocular surface disease is managed in the future.”

Obicetrapib: Consistent Lipid-Lowering, Silent Systemic Risks
Obicetrapib: Beyond Lipids
The BROADWAY Phase 3 trial confirmed obicetrapib’s potent LDL-lowering efficacy (−32.6%) in high-risk cardiovascular patients already on maximal statins. But when efficacy is statistical and safety is probabilistic, the real question shifts:
What don’t we see—yet?
While clinical endpoints are reassuring, CETP inhibition raises mechanistic flags beyond plasma cholesterol:
Could rigidified macrophage membranes impair immune presentation?
Might hepatic cholesterol be silently accumulating?
Is hormonal balance vulnerable to long-term precursor depletion?
As obicetrapib moves from trial to population, its safety narrative must evolve from absence of harm to proactive detection of subtle biological shifts—before they manifest as clinical signals.

From Enabler to Modulator: A Conceptual Framework for Enzyme-Controlled Subcutaneous Biologic Delivery
🔬 From Permeability to Programmability: A New Vision for Subcutaneous Drug Delivery
Most SC biologics today rely on hyaluronidase to open space, not control time. But what if the enzyme itself became the regulator?
This article introduces a strategic shift: embedding recombinant hyaluronidase within biocompatible matrices—not to enable volume, but to modulate release. The result? A programmable depot where antibodies, bispecifics, or even digoxin can be delivered with kinetic precision.
It’s not a patent. It’s a blueprint.
For those who think formulation isn’t just about convenience—it’s about control.

Mutation Without Smoke: How Urban Pollution Generates Genetic Signatures More Aggressive Than Tobacco
When pollution writes the genome.
In the largest study ever conducted on lung cancer in never-smokers, fine particulate pollution (PM2.5) was shown to trigger TP53 mutations—the same lethal genetic alterations caused by tobacco. Urban air doesn’t just irritate the lungs; it structurally reshapes the genome. As TP53-targeted immunotherapies emerge, we face a paradigm shift: pollution isn’t just a public health issue—it’s a mutagenic force with trillion-dollar implications for drug development, regulation, and justice. The data is no longer observational. It’s molecular.

Volume vs. Precision: What We Learned From Two Structurally Opposite Investigations
We often assume that the longer a report is, the harder it must be.
That’s false.
The real exhaustion — both for humans and machines — comes when logic must remain unbroken across diverse domains, with no margin for error.
When finance must align with ethics, and audit notes must reflect clinical protocol changes, volume becomes secondary to structural fidelity.
This is where synthetic systems stretch to meet human precision.
And this is where Biopharma Business Intelligence begins to expose the real fragility behind biotech narratives.

What Happens to Health During an Economic Collapse?
In Part 2 of our series on structural health vulnerability, we dive into the silent erosion of respiratory stability during economic downturns.
High male smoking rates, rising female tobacco use, poor disease understanding, and unaffordable controller therapies converge into a pulmonary time bomb — especially in winter.
We show why asthma and COPD aren’t just clinical issues — they’re system indicators.
When they spike, it's already too late.

Crisis Economics and Cardiovascular Collapse
When economies collapse, hearts break — not just figuratively, but biologically.
This article reveals how financial crises trigger a cascade of stress-driven physiological changes that culminate in strokes, heart attacks, and preventable deaths. Drawing from real-world data in Ireland, Greece, and the UK, and backed by a structured intervention model, it makes a simple case: public health systems must act before the body breaks — not after.

High-Precision Therapies vs. Collective Impact: The Case of Hemgenix and National Productivity
💡 What if curing disease isn't enough?
In 2025, the UK publicly funded Hemgenix — a $3.3M gene therapy for hemophilia B — offering life-changing benefits to a few dozen adults. But beneath the clinical triumph lies a structural question:
👉 Is this how we maximize collective return on public health investment?
This article explores the economic and ethical calculus behind breakthrough therapies, contrasting late-stage interventions with early, high-ROI strategies that preserve national productivity from childhood onward.
📊 From GDP modeling to risk-adjusted costs of lifelong transfusions, we analyze why innovation should align not just with individual outcomes, but with societal impact.

From Plastic to Paracetamol to Pavement: A Strategic Vision for Real Circular Value
What if we could turn plastic waste into medicine, energy and infrastructure—within one integrated, self-cleaning system?Not a proposal. A working model.

How a Clinical Trial Is Saved (or Ruined): Lessons from CTD Module 5
A critical breakdown of how clinical trial execution impacts the final CSR.
Based on direct experience, this piece walks through the CTD Module 5, SAE documentation, CRO collapse, CRA turnover, and why in-house oversight isn't optional.

CTD Module 4: The Cheapest Place to Prevent a $200M Loss
When companies talk about clinical development, most of the attention goes to the trial phases: Phase I safety, Phase II signals, Phase III failure or success.
But before all of that, there is a module—silent, technical, often overlooked—whose quality determines whether those millions should even be invested.
That module is CTD Module 4: Nonclinical Studies.
And its real value is not academic. It’s financial.

Mapping the Future of Big Pharma: Product Lifecycle, Upcoming Launches, and 5-Year Revenue Impact
In a rapidly shifting pharmaceutical landscape, five industry titans—Johnson & Johnson, Eli Lilly, Roche, Merck, and Pfizer—command both the present and the near future. This article dissects their current product portfolios, lifecycle dynamics, and late-stage pipelines, revealing which upcoming launches may offset blockbuster declines and reshape revenue forecasts through 2030. A must-read snapshot for those tracking the strategic evolution of global pharma.
