Ribociclib: From Phase I Dose Definition to Adjuvant Positioning — Clinical Trajectory and the Transparency Dilemma
Signals of resistance — documented years ago in FELINE and related preprints — remain underacknowledged in official narratives. The true credibility of adjuvant CDK4/6 therapy will not be secured by hazard ratios alone, but by full transparency on resistance, toxicity, and the patient experience. Only then can ribociclib’s full arc — from safe dose definition in phase I, exploratory validation in phase II, to pivotal MONALEESA survival gains and adjuvant confirmation in NATALEE — be properly understood as both achievement and unresolved dilemma
Adjuvant CDK4/6 Inhibitors in Early Breast Cancer: Benefit, Cost, and the Emerging Paradox of Resistant Relapses
Adjuvant use of CDK4/6 inhibitors in early breast cancer brings a paradox: modest reductions in recurrence at the cost of profound human and economic burdens. Abemaciclib prevents one relapse for every ~14 patients treated; ribociclib for every ~32. Yet the price is measured not only in millions of dollars per event avoided, but also in years of fatigue, gastrointestinal distress, liver toxicity, disrupted family life, and the silent erosion of mental health. For many survivors, the treatment does not end with the last pill—it leaves behind scars that shape work, relationships, and future prospects. Families facing this decision must balance time gained against suffering endured, with no certainty yet that overall survival is extended.
Sanofi’s $13B Erosion: Amlitelimab’s Trial Results and the Pipeline Dilemma
Sanofi’s $13B market loss following amlitelimab’s Phase III results reflects not a scientific failure, but a mismatch between biology and expectations. Unlike Dupilumab’s rapid downstream blockade of IL-4/IL-13, amlitelimab acts upstream at the OX40L–T cell axis, requiring more time to reshape immune responses. The COAST-1 trial met its primary endpoints using vIGA-AD, a more rigorous scale than Dupilumab’s IGA, and included adolescents as well as adults, making direct comparisons misleading. Efficacy at 24 weeks was statistically significant (21–23% vs. 9% placebo), safety was favorable, and long-term potential extends to synergy in atopic dermatitis and applications in autoimmune disease. The market judged amlitelimab as a weaker Dupixent; in reality, it is a different drug with a different biological logic.
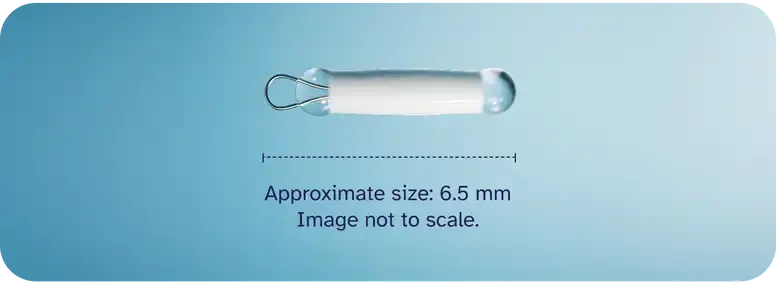
One Dose versus Three Doses of Benzathine Penicillin G in Early Syphilis (NCT03637660, NEJM 2025)
The NEJM 2025 trial (NCT03637660) has overturned a decades-old convention in syphilis treatment: a single 2.4M unit dose of benzathine penicillin G proved noninferior to the traditional three-dose regimen. This finding comes at a time when syphilis cases are resurging globally—and in South Korea, where nearly 2,800 cases in 2024 were concentrated among young men, reinforcing both a clinical and structural vulnerability. The lesson is clear: sufficiency, not redundancy, ensures resilience. Read more here.
Beta-Blockers Post-MI: Sex-Specific Evidence and the Collapse of a Universal Dogma
For decades, beta-blockers were given automatically after every heart attack. Modern evidence now shows they are not always necessary: they remain vital for patients with weakened heart function or arrhythmias, but offer no universal benefit. The future of cardiac care lies in personalized treatment, not one-size-fits-all dogma.
Harlem Legionnaires’ Outbreak: Structural Failures in Public Health Oversight
Legionnaires’ disease is a preventable but deadly pneumonia caused by Legionella pneumophila, a Gram-negative bacterium thriving in warm water systems and cooling towers. The Harlem outbreak revealed how underfunded surveillance and neglected infrastructure can transform hospitals and laboratories into bacterial amplifiers, exposing the structural fragility of urban health governance.
Live-Attenuated RSV Vaccine in Infants: Structural Integrity Assessment
Sanofi’s intranasal live-attenuated RSV vaccine (RSVt) showed strong safety and immunogenicity in infants, avoiding the pitfalls of past failed candidates. Targeting mucosal immunity through a high-dose strategy, RSVt could become the first scalable pediatric platform to reduce RSV hospitalizations and reshape early-life prevention
U.S. CDC Leadership Crisis: Structural Breakdown Under Political Pressure
The removal of CDC Director Susan Monarez and the resignation of four senior officials reveal a deeper conflict: a public health establishment historically aligned with pharma and universal vaccination now faces Robert F. Kennedy Jr.’s push for selective immunization. The struggle is less about science versus politics, and more about which model of legitimacy will guide U.S. health policy.
Encelto™ and Macular Telangiectasia Type 2: Structural Promise Without Functional Proof
Encelto™, the first FDA-approved therapy for macular telangiectasia type 2, demonstrates clear structural benefit by slowing loss of the ellipsoid zone on OCT. Yet at 24 months, patients showed no functional improvement in vision or reading compared with sham surgery. This approval, based on a surrogate endpoint, underscores a critical gap: histologic preservation does not equal clinical benefit. The true value of Encelto will depend on whether long-term Phase 4 data confirm that structural protection translates into meaningful visual function.
Pig-to-Human Lung Xenotransplantation: First Clinical Attempt and Structural Implications
The first pig-to-human lung xenotransplantation, performed in China and published in Nature Medicine, demonstrated temporary graft function in a brain-dead recipient for nine days. While technically notable, the study underscores unresolved challenges: predictable short survival despite maximal genetic editing and immunosuppression, opaque consent disclosure, and the ethical tension of using human bodies as experimental platforms.
Revolutionary Bioactive Laser Therapy for Post-Infectious Corneal Disease - DualStem-Cor™
DualStem-Cor™ is a pioneering protocol combining excimer laser ablation with the imprinting of dual-source autologous stem cells—ectodermal from buccal mucosa and mesodermal from peripheral blood—onto a therapeutic lens scaffold. Designed for patients with post-infectious corneal scarring and irregular astigmatism, it offers a pathway to restore vision and reduce dependence on corneal transplantation.
CDC Abandons Universal COVID Shot Guidance and Splits MMRV Schedule: A Structural Recalibration of U.S. Vaccine Policy
The University of Florida’s breakthrough in Nature Biomedical Engineering marks the emergence of a third paradigm in cancer vaccination: not personalized to each tumor, nor directed at a single antigen, but a generalized immune trigger delivered via mRNA. By amplifying type-I interferon responses, tumors once invisible to the immune system became targets, even showing eradication in mouse models. Yet translation to humans hinges on two unresolved frontiers—containing systemic toxicity through precise nanoparticle targeting, and safeguarding patients against retroviral or retroelement activity that could enable unintended genomic interactions. Without these safeguards, the “universal cancer vaccine” remains a prototype; with them, it could reshape the architecture of oncology itself.
Molgramostim in Pulmonary Alveolar Proteinosis
The IMPALA-2 phase 3 trial (NEJM, 2025) establishes inhaled molgramostim as the first effective pharmacologic therapy for autoimmune pulmonary alveolar proteinosis (aPAP). In 164 randomized patients, molgramostim achieved a +9.8% improvement in DLCO at 24 weeks versus +3.8% with placebo, with benefits increasing to +11.6% at 48 weeks. While placebo saline nebulization itself improved gas transfer, molgramostim demonstrated a robust and biologically coherent effect by restoring alveolar macrophage function impaired by GM-CSF autoantibodies.
Unlike systemic GM-CSF (sargramostim), molgramostim is a non-glycosylated recombinant protein formulated for alveolar delivery, achieving high local exposure with minimal systemic toxicity. This pharmacokinetic advantage, together with its orphan-drug positioning, projects a market entry price in the US$200,000–700,000 annual range. Beyond efficacy, molgramostim signals a paradigm shift: from invasive whole-lung lavage to chronic inhaled immunomodulation in rare lung disease.
Radioactive Shrimp Recall at Walmart: FDA Intervention, Supply Chain Vulnerabilities, and Consumer Risk Signals
The FDA’s recall of Great Value shrimp sold at Walmart is not a minor food safety incident but a structural warning: cesium-137, a radioactive isotope with a 30-year half-life, combines internal beta damage with systemic gamma irradiation. Even when measured below intervention thresholds, repeated dietary exposure compounds genetic risk. Without true “boat-to-plate” traceability, consumers cannot distinguish a safe lot from one captured in a radioactive hotspot. In such radical uncertainty, overreaction is not hysteria—it is the only rational defense against decades of hidden health costs and the collapse of public trust.
CagriSema (cagrilintide + semaglutide) – REDEFINE 1 and REDEFINE 2 Trials: 6 deaths, all in active arms; BBIU warns of the need for intensive post-marketing surveillance
⚠️ BBIU Warning:
Although the six deaths reported in the REDEFINE trials were adjudicated as not related to treatment, the fact that all occurred in active arms justifies intensive post-marketing surveillance to rule out emerging risk patterns in real-world practice.
Mortality references in the REDEFINE trials
REDEFINE 1 – Adults with overweight or obesity without diabetes
Reference: Garvey T, Blüher M, Contreras C, et al. Coadministered Cagrilintide and Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2025; DOI: 10.1056/NEJMoa2502081
Report: 2 deaths in the CagriSema arm, adjudicated as not related to the drug (suicide and cancer of unknown primary). Determination made by independent committee.REDEFINE 2 – Adults with overweight or obesity and type 2 diabetes
Reference: Davies M, Bajaj H, Broholm C, et al. Cagrilintide–Semaglutide in Adults with Overweight or Obesity and Type 2 Diabetes. N Engl J Med. 2025; DOI: 10.1056/NEJMoa2502082
Report: 4 deaths in the CagriSema arm, adjudicated as not related to treatment, attributable to underlying causes.
[Syphilis Cases in South Korea Concentrated in Young Males: Epidemiological Trends and Structural Implications]
In Korea, the apparent 2024 “surge” in syphilis is less a new outbreak than the exposure of a chronic, under-measured problem. The shift from sentinel surveillance to nationwide mandatory reporting revealed a long-standing burden, concentrated among young men, that has been fueled by gaps in practical sexual education, limited prevention culture, and selective health screening of migrants. Surveillance improvements provide sharper data, but without parallel investment in behavior change, universal STI prevention, and coherent border health protocols, the underlying transmission chains will remain intact.
🟢 [Cardiovascular Adverse Events and Costs in CDK4/6 Inhibitors – Real-World Evidence]
The JNCCN article (May 2025) provides real-world evidence on comparative cardiovascular risk (hypertension and MACE) among the three CDK4/6 inhibitors and the incremental economic impact of these events.
MACE incidence (per 100 PY):
Ribociclib 23.0
Abemaciclib 25.1
Palbociclib 18.3
Adjusted hazard ratio vs ribociclib:
Palbociclib 0.636 (significant)
Abemaciclib 0.795 (not significant)
Incremental PPPM costs:
Hypertension: +$2,964 (mainly outpatient and inpatient care)
MACE: +$4,010 (outpatient +$3,149, inpatient +$970)
Palbociclib shows a lower real-world risk of MACE compared to ribociclib. Ribociclib maintains the most unfavorable profile, associated with QTc prolongation and arrhythmic risk. Abemaciclib shows no relevant QTc signal but presents indirect cardiovascular risk due to gastrointestinal toxicity and electrolyte disturbances.
🟡 [GLP-1 Agonists and Sudden Vision Loss: Emerging Risk Signal Amid Global Weight-Loss Drug Boom]
While recent observational studies suggest a possible association between GLP-1 receptor agonists and non-arteritic anterior ischemic optic neuropathy (NAION), results remain heterogeneous — with some cohorts showing elevated hazard ratios and others finding no significant signal. NAION’s pathophysiology, rooted in compromised perfusion of the optic nerve head via the posterior ciliary arteries, supports biological plausibility, yet most documented cases involve patients with pre-existing vascular or systemic comorbidities rather than entirely healthy individuals. Symptom onset in case reports often occurs within weeks to three months of therapy initiation, but population-level data have not validated a discrete early-phase risk window. Given the irreversible nature of NAION and its poor visual prognosis, targeted surveillance, patient risk stratification, and informed consent are warranted while awaiting more definitive evidence.
From Thalidomide to Ribociclib: Why Approving Without Mature Data Can Repeat History
The NATALEE trial presents a regulatory dilemma reminiscent of the thalidomide case. While ribociclib shows a statistically significant improvement in invasive disease-free survival (iDFS) in early-stage HR+/HER2– breast cancer, the absolute benefit is modest (+3.1–3.3 points), toxicity is substantial, and overall survival data remain immature. In a potentially curable population, this benefit–risk balance does not justify mass exposure to serious adverse events and high costs. History shows that approving too early is easy—reversing late is devastating.
🟡 [Ousted FDA Vaccine Chief Returns to Agency – Vinay Prasad’s Unusual Rehiring]
The reinstatement of Dr. Vinay Prasad to lead the FDA’s Center for Biologics Evaluation and Research marks a rare reassertion of scientific authority over political pressure. Backed by Health Secretary Robert F. Kennedy Jr. and FDA Commissioner Marty Makary, Prasad’s return signals resistance to both industry capture and ideological loyalty tests — setting the stage for a more confrontational regulatory environment.















![[Syphilis Cases in South Korea Concentrated in Young Males: Epidemiological Trends and Structural Implications]](https://images.squarespace-cdn.com/content/v1/685a879d969073618e9775db/1755226938811-SHNZB1KR36CWW2ZQXW7J/0003462182_001_20250815094113000.jpg)
![🟢 [Cardiovascular Adverse Events and Costs in CDK4/6 Inhibitors – Real-World Evidence]](https://images.squarespace-cdn.com/content/v1/685a879d969073618e9775db/1755139598612-W7GG7SZV5SY0IYP0DUAH/cancer-mama-900x450-1-qwy7a38kddol2dblrik5f94rrjxfuups7nwivhl17i.jpg)
![🟡 [GLP-1 Agonists and Sudden Vision Loss: Emerging Risk Signal Amid Global Weight-Loss Drug Boom]](https://images.squarespace-cdn.com/content/v1/685a879d969073618e9775db/1755057272792-C95PY08O44BLJLIBLM4Y/retina_bloodvessels_642.jpg)

![🟡 [Ousted FDA Vaccine Chief Returns to Agency – Vinay Prasad’s Unusual Rehiring]](https://images.squarespace-cdn.com/content/v1/685a879d969073618e9775db/1754795518545-20TB7G0VJBJ1YAJNO5MV/09dc-prasad-wtpz-superJumbo.webp)